CBD Öl bzw. Cannabisöl kaufen ist mittlerweile, im wahrsten Sinne des Wortes, in aller Munde. Dies hat auch seinen Grund, denn CBD Öl ist absolut vielseitig und kann bei den verschiedensten Krankheiten und Beschwerden hilfreich unterstützen und lindern. Aus diesem Grund wurde auch das Betäubungsmittelgesetz im März 2017 gelockert und Ärzten ist es nun gestattet, medizinisches Cannabis zu verschreiben.
Wo man CBD Öl kaufen kann: Die besten Shops
| Shop | Auswahl | Bewertung | |
|---|---|---|---|
 Nordic Oil |
|
| 9,6/10 |
 CBD Vital |
|
| 9,4/10 |
 cbdkaufen.com |
|
| 9,2/10 |
 BioCBD |
|
| 8,9/10 |
 CBDwelt.de |
|
| 8,7/10 |
 Hempamed |
|
| 8,1/10 |
 Alpinols |
|
| 7,6/10 |
 Elixinol |
|
| 7,1/10 |
Was ist CBD?
CBD kommt wie auch THC in der Hanfpflanze vor und wurde schon von vielen Völkern als Heilmittel genutzt. Die Abkürzung CBD steht dabei für Cannabidoil, ein Wirkstoff, der aus der weiblichen Hanfpflanze oder direkte aus den weiblichen Hanfsamen Cannabis sativa oder Cannabis indica extrahiert wird. Auch das “high” machende THC kommt in der Hanf Pflanze vor und wird daher oft fälschlicherweise in Verbindung mit CBD oder Cannabis Öl gebracht.
Mittlerweile sind aufgrund der breiten Akzeptanz von Cannabis auch Hanfsorten gezüchtet worden, die frei von THC sind und somit auch ein vollkommen THC-freies Hanfprodukt hergestellt werden kann.
Im Gegensatz zum THC, wirkt CBD nicht psychoaktiv oder berauschend und fällt somit nicht unter das Deutsche Betäubungsmittelgesetz.
Cannabis kaufen ist und bleibt weiterhin verboten, der Kauf von Cannabis Öl in Deutschland als Nahrungsergänzungsmittel oder Kosemtikprodukt ist mittlerweile hinigegen legal und unproblematisch, solange der THC Gehalt einen Wert von 0,2% pro Einheit nicht übersteigt.
CBD Gehalt in Cannabis Öl
Die Konzentration von CBD in Cannabis Öl sagt viel über die Wirkung und den Nutzen für den Anwender aus. In der Regel wird der CBD-Gehalt in Prozent angegeben und bezieht sich auf das Verhältnis von CBD in Milligramm zur gesamten Menge in Milliliter des Behältnisses. Die bekanntesten Marken bieten Cannabis mit Konzentrationen zwischen 1% und 25% an, die gängigsten CBD Öle beinhalten 5%, 10% oder 20%.
Dabei hat der Kauf von CBD Öl eine so vielseitige Wirkung, dass es es bei Depressionen ebenso zum Einsatz kommt, wie auch bei Krebs, um die Nebenwirkungen der Chemotherapie zu lindern. Weiterhin kann es bei Epilepsie, Multiple Sklerose, Alzheimer und vielen weiteren Krankheiten als unterstützende Therapie ein wichtiger Helfer sein. Selbst Kinder und Haustiere können von Cannabidiol profitieren.
Natürlich ersetzt der Kauf und die Einnahme von CBD Öl keine gesunde und ausgewogene Ernährung, dennoch kann das Immunsystem gestärkt werden. Wer beispielsweise den Geschmack von CBD nicht mag, kann die Tropfen auch einfach in seinen Smoothie oder Saft geben. Mahlzeiten mit einem hohen Flüssgkeitsgehalt lassen sich ideal mit dem gekauften CBD Öl anreichern und der oft als unangenehm empfundene Geschmack abgemildert werden, zu beachten ist jedoch, dass hier runter auch die Wirksamkeit leiden kann. Nach einer Weile gewöhnt man sich an den Geschmack und der CBD Öl Kauf wird zu einer regelmäßigen Sache.
Vorteil hierbei ist, dass CBD Öl keinen Rausch, wie THC, auslöst und somit nicht psychoaktiv wirkt. Es ist außerdem legal erhältlich und wird mittlerweile von zahlreichen Menschen genutzt. Dabei ist erstaunlich, dass viele Menschen über 50 Jahre von dem Cannabidiol absolut begeistert sind und es uneingeschränkt weiterempfehlen.
CBD Öl kaufen: Anwendungsgebiete
Cannabidiol kann sehr vielfältig genutzt werden. Dabei ist es nicht nur bei Schlafstörungen und Stress ein wichtiger Helfer, sondern auch bei Autismus und Epilepsie kann CBD Öl hilfreich wirken. Zudem kann es bei folgenden Krankheiten und Beschwerden zum Einsatz kommen:
- Morbus Crohn
- Alzheimer und Parkinson
- Multiple Sklerose
- ADS/ADHS
- Schizophrenie
- Chronisch entzündliche Krankheiten
- Depressionen
- Krebs
Sogar bei Hautkrankheiten wie Akne kann das Cannabidiol unterstützend wirken. Einen ausführlichen Bericht über CBD Öl und seine Anwendungsgebiete gibt es in dem Unterpunkt Anwendungsgebiete.
Aktuell gibt es eine Sonderaktionen auf Nordic Oil “3 für 2 Angebote“: Verwende folgenden Rabattcode: 3FOR2 im Warenkorb.
CBD Öl kaufen – Der Geschmack
Wer Cannabis Öl nicht nur äußerlich anwenden will, sondern seiner Gesundheit durch den Konsum von CBD etwas gutes tun möchte den interessiert natürlich wie sich die wenigen Tropfen auf den Geschmack des Smoothies, Müslis oder der Mahlzeit auswirken.
Der Geschmack von CBD Öl hängt von der Herstellungsweise, dem Trägeröl und der Konzentration des Cannabidiol, den enthaltenen Terpenen und Flavonoiden ab. Vor allem die Terpenen enscheiden über den Geschmack, dementsprechend kann der CBD Öl Kauf für Überraschungen sorgen. Ein breites Geschmacksspektrum von fruchtig bis herb kann hierbei auftreten. Auch das Trägeröl wirkt sich auf den Geschmack aus und kann bei Hanföl einen intensiv würzigen, bei Avocadoöl für einen milden Geschmack und bei einer Kokos oder Sonnenblumenöl basis für einen gewissen intensiven Geschmack sorgen.
Die Farbe der CBD Öle
Wer Cannabis Öl kaufen möchte, der wird früher oder später darauf aufmerksam, dass verschiedene CBD Öle verschiedene Farben habe. Diese reichen von sehr hell, über goldgelb bis zu einem dunklen braun. Oft stellt sich die Frage ob sich hier Rückschlüsse auf Qualität oder Haltbarkeit ergeben – dies können wir entschiedenen verneinen, ein dunkel gefärbtes CBD Öl bedeutet nicht das dieses schlecht oder nicht mehr wirksam ist!
Vielmehr entscheidet wieder rum das Trägeröl und auch die eingesetzte Cannabissorte über die Färbung. Wird Hanföl als Träger verwendet tritt häufig eine hellbraune bis gründliche Färbung auf, nutzt man hingegen Kokosöl färbt sich das CBD Öl sehr hell und in manchen Fällen sogar weiß.
Cannabis Öl Kaufen Produkt und Preisvergleich 2020:
Cannabis Öl kaufen – Welche Ausführungen gibt es?
Als organische Verbindung, auch Pytocannabinoide (oder einfach Cannabinoide) genannt, besitzt CBD die Fähigkeit im menschlichen Körper bestimmte Rezeptoren anzusprechen und somit aktiv Einfluss auf das Wohlbefinden zu nehmen.
Ein entscheidendes Unterscheidungsmerkmal ist das Cannabinoid-Spektrum.
Unterschieden wird hier in zwei verschiedene Arten, auf der einen Seite das sogenannte Vollspektrum CBD Öl, mit einem vielfältigen Cannabinoid Profil und einer hohen Anzahl von Flavonoiden und Terpenen. Beim Vollspektrum CBD Öl ergänzt sich die Werkstoffmatrix im Idealfall und führt zu einer stärkeren Wirkung.
Auf der anderen Seite gibt es das CBD Isolat, welches sich durch eine sehr hohe Konzentration von reinem CBD auszeichnet und von zusätzlichen Flavonoiden oder anderen Stoffen gereinigt wurde. Meist lässt sich CBD Isolat im Gegensatz zu CBD Öl in Kristallform kaufen und einnehmen.
Unterschiede zwischen Vollspektrum CBD Öl und CBD Isolat
Während ein Vollspektrum CBD Öl das volle Spektrum an Cannabinoiden, Terpenen und Flavonoiden, und unter umständen auch eine geringe Menge THC enthält. Ist das CBD Isolat die reine Form von CBD.
CBD Öl aus Vollspektrum CBD Extrakt mit einem Zusammenspiel von z. B.
- CBD
- CBDa
- CBN
- CBC und CBG
bietet den Vorteil, dass sich die positiven Effekte durch das natürliche Verhältnis im Idealfall begünstigen. Die Wirkung des CBD-Öls im Körper entfaltet sich leichter und vielfältiger. Vollspektrum-Öle sind in Allgemeinen wirksamer und daher auch empfehlenswerter als der CBD Öl Kauf von CBD Isolat. Nicht nur in der Wirkung sondern auch im Geschmack, Geruch und der Farbe lassen sich vollwertige CBD Öle unterscheiden. Vollspretrum CBD Öl enthält mehrer Bestandteile der Hanfpflanze und ist somit deutlich intensiver im Geschmack und intensiver im Geruch. Auch an der Farbe erkennt man es. Vollspektrum CBD Öl ist generell dunkler gefärbt. Hellere CBD Öle sind reiner und stärker filtriert und somit auch milder im Geschmack.
Vergleichstabelle: Vollspektrum vs. Isolat CBD
| CBD Öl Vollspektrum | CBD Isolate (Kristalle) | |
| Cannabinoid-Spektrum | Beinhaltet viele verschiedene Cannabinoide | Enthält reines CBD |
| THC Gehalt | geringer Anteil THC enthalten | Kaum bis kein THC |
| Wirksamkeit | Stärkere Wirkung | Geringere Wirkung |
| Geruch | milder, natürlicher Geruch | kaum Geruch |
| Geschmack | Intensiver Geschmack durch natürlich vorkommende Terpenen | Geschmacksneutral – kaum bis kein Eigengeschmack |
| Nebenwirkungen | Selten | Häufig |
| Synergie-Effekt | Begünstigt durch natürliche Form | Keiner durch starke Filtration |
Wie wirkt CBD Öl (Cannabis Öl) ?
Wer CBL Öl kaufen möchte fragt sich logischerweise wie genau der Wirkstoff eigentlich im Körper wirkt. Zwar sind die positiven Effekte weitgehend unbestritten und auch von Studien untermauert, dennoch bleibt viele offene Fragen bezüglich der Wirkung und Effektivität von Cannabis Öl. Die Tatsache, dass jeder Organismus unterschiedlich auf die CBD Wirkstoffe reagieren kann erlaubt keine pauschalen Aussagen, die meisten Nutzer von CBD Öl berichten aber von spürbar positiven Effekten.
Zusammenhänge zwischen CBD Ölen und dem damit einhergehenden körperlichen Wohlbefinden können mit der Aktivierung des sogenannten Endocannabinoid Systems erklärt werden.
Cannabis Öl und das ECS System
Nachdem das körpereigene Endocannabinoid System Anfang der 90er im menschlichen Körper entdeckt wurde, wurden die Cannabidiole verstärkert erforscht, und eine direkte Interaktion mit dem Endocannabinoid System (kurz ECS) nachgewiesen. Vor allem im Bereich der muskulären Verspannungen scheint das ECS direkten Einfluss auf das körperliche Wohlbefinden zu nehmen.
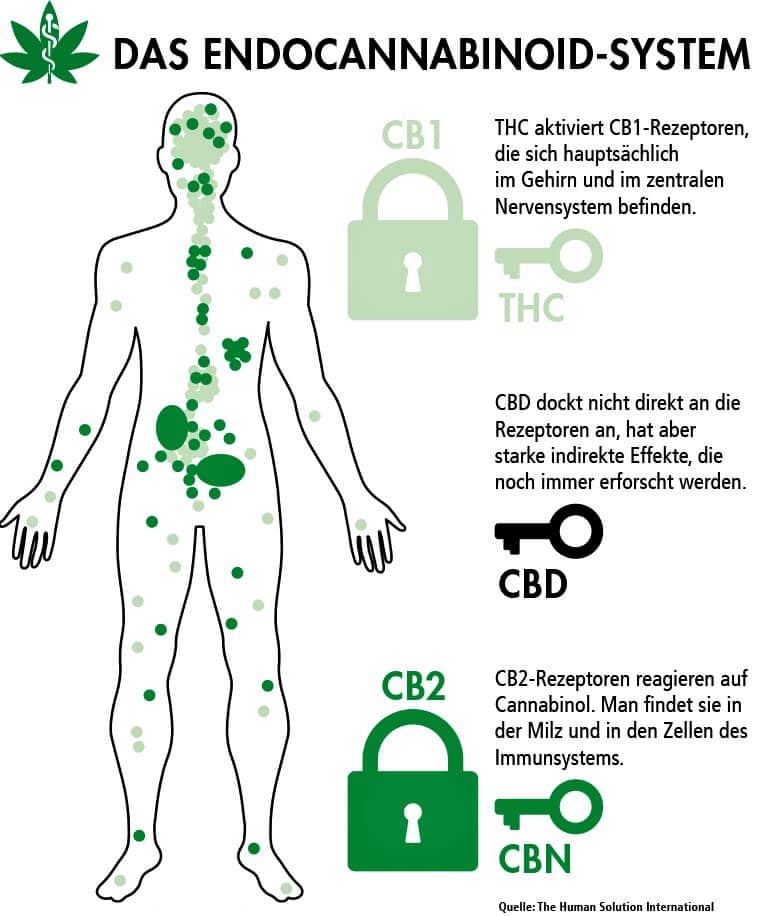
ECS System – Funktionsweise
CBD dockt, anders als das psychisch wirkende THC, vor allem an den Muskelrezeptoren an und führt somit den entspannten Zustand herbei von dem Kunden die einen Cannabis Öl Kauf getätigt haben häufig berichten.
Allgemeinhin wird davon ausgegangen, dass das Encdocannabinoide System direkten Einfluss auf
- Das Stimmungsbild und Stressbedingte Symptome wie z.B. Muskelverspannungen, Angstzustände oder Depressionen hat
- Den gesamten Schlafprozess
- Eine Vielzahl von Immunfunktionen
- Die Verdauungsfunktionen
- und weitere Körpereigene Funktionen
hat.
Cannabis Öl aktiviert das Endocannabinoide-System
CBD gilt als ein sehr komplexes Cannabinoid, das das Endocannabinoidsystem stimulieren und zur Ausschüttung des Glückshormons Serotonin beitragen kann. Ein Mangel an Serotonin wird oft mit Depressionen in Verbindung gebracht. die Vermutung steht also nahe, dass sich mit CBD ÖL Depressionen lindern lassen!
Die Behauptung wird durch die Beobachtung untermauert, dass die Symptome häufig durch eine Erhöhung des Serotoninspiegels gelindert werden können.
Ein bedenklich niedriger Gehalt an Endocannabinoiden im Körper wird von Wissenschaftlern inzwischen als möglicher Auslöser für mehrere psychische und physische Krankheiten angesehen. Der Endocannabinoid-Spiegel kann bei bestimmten Aktivitäten ansteigen. Zum Beispiel produziert unser Körper beim Sport, Singen oder Tanzen mehr Endocannabinoide. Das ist auch der Grund dafür, dass wir uns während dieser Aktivitäten besser fühlen, unsere Stimmung verbessert und unser Stresspegel sinkt.
Die exogenen Cannabinoide, zugeführten Cannabinoide, also auch CBD-Öle aktivieren ebenfalls das Endocannabinoid-System. Dies kann die Wahrscheinlichkeit erhöhen, sich entspannter, gelassener und allgemein wohler fühlen. CBD kann z.B. sehr gut als Lebensmittelzusatzstoff, als Massageöl und zur direkten Einnahme verwendet werden. Viele CBD-Öl Käufer berichten im Zusammenhang mit der regelmäßigen Anwendung von einer durchweg verbesserten Stimmung, mehr Entspannung im alltäglichen Leben, einer besseren Grundstimmung und einer besseren Bewältigung der täglichen Probleme.
Wirkungsdauer und Wirkungseintritt von CBD Öl
Cannabis Öl kann auf verschiedene Arten und in verschiedenen Formen eingenommen werden. Entscheidend für die Wirkungsdauer und auch den Wirkungseintritt nach Einnahme ist die Art der Einnahme. Die bekannteste und beliebteste Form der Einnahme von CBD-Öl ist die orale Einnahme durch Tropfen mithilfe einer mitgelieferten Pipette die bei den meisten Anbietern enthalten ist, wenn man Cannabis Öl kauft.
Bei der direkten Einnahme des CBD Öl gelangt der Wirkstoff direkt über die Mundschleimhaut schnell in den Blutkreislauf und entfaltet seine Wirkung sehr schnell. Im Idealfall sollte es “sublingual” genommen werden. Das bedeutet, dass die CBD-Tropfen unter die Zunge getropft werden und dort bis zu 60 Sekunden verbleiben, bevor sie normal geschluckt werden. Dies hat den Grund, dass sich unter der Zunge zahlreiche Blutgefäße befinden, über die eine Aufnahme des CBD direkt erfolgen kann, die unmittelbare und effektive Absorption erhöht ebenfalls die Bioverfügbarkeit. Wird das CBD Öl hingegen normal geschluckt wird einiges des Wirkstoffes im Magen zerstört und nur in geringer Menge vom Körper aufgenommen, die Wirkung wird somit verringert.
Bis zur Vollständigen Aufnahme des CBD-Öls dauert es ca. 40-75 Minuten, der Beginn der Wirkung ist bereits nach 15, spätestens jedoch nach 60 Minuten spürbar. Die Wirkungsdauer kann etwa 4-6 Stunden dauern
Wenn das CBD-Öl direkt oder mit der Nahrung aufgenommen wird, dauert es etwas länger, bis das CBD-Öl in den Blutkreislauf gelangt.
| Produkt | Wirkungseintritt | Wirkungsdauer |
| CBD Öl | Bereits nach wenigen Minuten | 4-6 Stunden |
| CBD Liquids | Unmittelbar nach Aufnahme | ungefähr 4 Stunden |
| CBD Salben / CBS Creme | ca. 60 Minuten nach Auftragen | ungefähr 6 Stunden |
CBD Öl Einnahme
Beim CBD Öl Kauf sollte vor allem darauf geachtet werden, dass die Flasche eine Dosierungspipette enthält. Hiermit lässt sich die gewünschte Anzahl von Tropfen direkt auf oder unter die Zunge träufeln. Wie bereits erwähnt ist mit dieser Form der Verabreichung die Aufnahme optimal und die Wirkung entfaltet sich in kürzester Zeit.
Auch über die Haut kann CBD Öl aufgenommen werden, allerdings dauert es um einiges länger bis sich eine vollständige Wirkung entfaltet, zusätzlich ist die Wirkung deutlich abgeschwächt und die lokale Wirkung von CBD Cremen und Salben umstritten.
CBD Öl Dosierung
Abhängig davon ob es sich um ein CBD Öl mit 5%, 10%, 15% oder 20% handelt, wird die Dosierung entsprechend angepasst.
| Menge | ideale Kontentration im CBD Öl | Einsatzbereich |
| 20mg Cannabidiol pro Tag | 2% – 8% | Vorbeugung und Gesundheit |
| 20-100mg Cannabidiol pro Tag | 10% – 20% | kleine Beschwerden |
| über 100mg Cannabidiol pro Tag | min. 20% | schwere Krankheit |
Step Up Methode
Eine bekannte und weit verbreitete Methode um die richtige Dosierung zu ermitteln ist die sogenannte Step-up Methode. Bei dieser simplen Methode wird die Dosis an CBD Öl solange erhöht, bis die subjektiv wahrgenommene ideale Wirkung eintritt.
Beispielhaft kann man sich an dieser Rechnung orientierten, ausgehend von einer Einnahme 3x am Tag:
- Tag: einen Tropfen (morgens, mittags oder abends) = 1 Tropfen
- Tag: einen Tropfen morgens & einen Tropfen abends = 2 Tropfen
- Tag: einen Tropfen morgens, einen mittags & einen abends = 3 Tropfen
- Tag: zwei Tropfen morgens, einen mittags & einen abends = 4 Tropfen
- Tag: zwei Tropfen morgens, einen mittags & zwei abends = 5 Tropfen
- Tag: zwei Tropfen morgens, zwei mittags & zwei abends = 6 Tropfen
Der entscheidende Vorteil dieser Methode besteht darin, dass sich der Körper an den “neuen” Wirkstoff CBD gewöhnen kann und man somit seine persönlichen perfekte Dosis finden kann.
CBD Öl kaufen: Nebenwirkungen
Das Cannabidiol hat in der Regel keine Nebenwirkungen, wenn es sachgemäß verwendet wird. Lediglich wenn die Dosis zu hoch ist, können Nebenwirkungen auftreten. Diese zeigen sich häufig in Müdigkeit, Übelkeit und Erbrechen, Durchfall oder Schwinde. Auch Kopfschmerzen können vereinzelt auftreten.
Welche Personen auf das Cannabidiol verzichten sollten, wird im Artikel Nebenwirkungen vom CBD Öl Kauf ausführlich behandelt.
Cannabidiol ist ein wirklich vielfältiger Wirkstoff, der für die ganze Familie, inklusive Haustiere geeignet ist. Er wirkt nicht nur schmerzlindernd, sondern unterstützt auch das Immunsystem, wirkt sich auf die Gedächtnisleistung und die Konzentration aus und kann bei schweren Erkrankungen die schulmedizinische Therapie unterstützen.
Aus diesem Grund wäre es natürlich einen Versuch wert, bevor auf Pharmazeutika zurückgegriffen wird. Wie in Studien bewiesen wurde, kann der Konsum von Schmerzmitteln deutlich reduziert werden, wenn alternativ CBD Öl eingenommen wird. Allein dies ist schon ein Grund, es zumindest mit dem Cannabidiol zu versuchen, denn wirkt es nicht, kann es auch nicht schaden!
CBD Öl Vital und Nordic Oil bieten hervorragende Produkte in diesem Bereich und können sich zurecht als Marktführer auf dem deutschen Markt bezeichnen. Auch Hanafsan ist eine persönlicher Favorit. Da ich einige Öle ausprobieren möchte, kann ich euch in Zukunft mit Sicherheit einige Empfehlungen aussprechen oder auch sagen, welche Öle nicht so toll sind.
Außerdem findet ihr hier Gutscheine für CBD Produkte, die ich alle kenne und selbst probiert habe. Heißt also, dass ich sie absolut empfehlen kann. Auch wenn es noch nicht für alle Produkte einen Bericht gibt, könnt ihr sicher sein, dass ich auf meiner Seite nur Produkte empfehle, die ich selbst auch nehme.
CBD Öl Kaufen – Die Inhaltsstoffe
Die genauen Inhaltsstoffen unterscheiden sich mitunter stark von Anbieter zu Anbieter und hängen oft direkt von der Zusammensetzung des Trägeröls und dem eingesetzten CBD Extrakt ab (Vollspektrum oder CBD Isolat).
So enthält das Nordic Oil CBD Öl 15% neben 15% CBD auch Hanfsamenöl als Träger, Omega 3 und Omega 6 Fettsäuren und Vitamin E. Zusätzlich verbleiben im Cannabis Öl die natürlichen Terpene der Hanfpflanze, vorausgesetzt es wird ein Vollspektrum CBD Extrakt genutzt.
Zu beachten ist immer, dass in einigen Produkten ein geringer Anteil an THC verbleiben kann. Für in Deutschland zugelassenes CBD Öl darf dieser THC-Gehalt nicht über 0,2% liegen. Eine so geringe Menge an THC führt unter normalen Umständen zu keinem Rauschzustand, kann jedoch vor allem am Flughafen zu Probleme führen. Einige Länder haben deutlich strengere Richtlinien und verbieten Cannabis Öl kaufen auch mit einem sehr geringen Anteil an THC. Auch bei einem Drogentest können geringe Spuren von THC nachgewiesen werden und für eine Menge Ärger sorgen. Um dies zu vermeiden, lohnt sich der Cannabis Öl Kauf mit 0,0% THC greifen.
Trägeröle für Cannabis Öl
Die am häufigsten eingesetzten Trageröle sind auf der Basis von Hanfsamen-Öl, Avocadoöl oder Olivenöl hergestellt. Diese Trägeröle haben nicht nur die Aufgabe für eine geschmeidige Konsistenz zu sorgen, sondern sollen vor allem die Resorption, also die Aufnahme der Wirkstoffe im Körper fördern:
Die drei wichtigsten Hauptgründe für CBD Trägeröle sind:
- Trägeröle verbessern die CBD-Absorption
- Trägeröle erleichtern die Dosierung
- Trägeröle beinhalten wertvolle Inhaltsstoffe
Weshalb wirkt CBD Öl nicht berauschend?
Im Gegensatz zu THC wirkt CBD Öl nicht berauschend. Auch Cannabidiol Produkte, die THC enthalten lösen keinen Rausch aus, da der Anteil viel zu gering ist. Zudem heben sich beide Stoffe, also CBD und THC auf, da sie beide an die Rezeptoren andocken und sich somit in gewisser Weise einen Kampf liefern.
CBD geht dabei als stärkerer Wirkstoff hervor. THC muss durch das Andocken an die Rezeptoren kämpfen und verliert somit, während seines Kampfes, immer mehr an Kraft, weshalb somit CBD die Oberhand bekommt. Dies heißt gleichzeitig auch, dass THC nicht mehr so wirkungsvoll ist und der Rausch beim CBD Öl unterdrück wird.
CBD Öl kaufen für Kinder
CBD hat den großen Vorteil, dass sogar Kinder von dem Wirkstoff profitieren. Insbesondere bei ADS und ADHS hat sich das Cannabidiol bewährt und in vielen Foren wird berichtet, dass die Kinder, die CBD Öl kaufen ganz ohne Ritalin auskommen. Weiterhin fördert das Cannabidiol die Konzentration und soll auch die motorischen Fähigkeiten verbessern.
Mehr Informationen gibt es in dem Artikel CBD Öl kaufen für Kinder.
CBD Öl kaufen für Haustiere
Selbst Haustiere haben einen Vorteil von dem Cannabidiol, da es nicht nur für ältere Tiere, die unter chronischen Schmerzen leiden, anwendbar ist. Auch junge, ängstliche Tiere können von CBD Öl profitieren, da der Wirkstoff beruhigend wirkt. Aus diesem Grund kann Cannabidiol auch Tieren helfen, die den Weg über die Regenbogenbrücke gehen müssen.
Im Artikel CBD Öl kaufen für Haustiere gibt es viele weitere Infos.
CBD Öl bzw. Cannabis Öl Kaufen FAQ:
✅ Was ist CBD bzw. Cannabidiol?
Es handelt sich hier um einen Wirkstoff, der in der Cannabis Pflanze gefunden wird.
✅ Ist CBD Öl Kaufen in Deutschland legal?
Der Konsum von Cannabidiol (CBD) ist in Deutschland legal. Das entsprechende Produkt darf jedoch nicht mehr als 0,2% THC aufweisen.
✅ Ist CBD Öl das Gleiche wie Cannabis Öl?
Aus der Hanfpflanze wird Cannabis Öl gewonnen. Häufig wird das Cannabis Öl mit CBD Öl verwechselt. Es handelt sich jedoch um unterschiedliche Öle.
✅ Zu welchen Krankheiten oder Beschwerden kann CBD Öl zum Einsatz kommen?
Das Produkt kann sehr vielfältig eingesetzt werden. Beispielsweise bei Autismus oder Epilepsie kann das Öl hilfreich wirken. Zudem kann es bei zahlreichen Krankheiten oder Beschwerden wie Krebs, ADS/ADHS, Depressionen usw. zum Einsatz kommen.
| Shop | Auswahl | Bewertung | |
|---|---|---|---|
 Nordic Oil |
|
| 9,6/10 |
 CBD Vital |
|
| 9,4/10 |
 cbdkaufen.com |
|
| 9,2/10 |
 BioCBD |
|
| 8,9/10 |
 CBDwelt.de |
|
| 8,7/10 |
 Hempamed |
|
| 8,1/10 |
 Alpinols |
|
| 7,6/10 |
 Elixinol |
|
| 7,1/10 |


